|
As far as we know, there are four fundamental forces in the universe.
In order of increasing strength, they are:-
- The Gravitational force
(very weak, only noticeable when large
masses are involved)
- The Electromagnetic force
(responsible for electrostatic attraction
& repulsion,
also the behaviour of magnets)
- The Weak Nuclear force
(actually pretty strong, but only operates
over very short distances. Responsible for radioactive decay)
- The Strong Nuclear force
(very strong, but very short range. Responsible
for nuclear reations such as fission).
Inside any nucleus, the protons and neutrons are held together
by the incredibly powerful "Strong Nuclear Force", which
overcomes the electrostatic repulsion between the protons.
A balance exists between these two forces.
This means that certain numbers of protons and neutrons can make
a stable nucleus, whilst other groupings will be more or less unstable.
These will eventually decay to produce a more stable arrangement.
As the Atomic Number (that's the number of protons) increases,
atoms seem to need more neutrons.
Carbon-12 has 6 protons and 6 neutrons, whilst Uranium-238 has 92
protons and 146 neutrons.
Once we get more than 82 protons (that's Lead), the nuclei are no
longer stable, and we're into radioactive elements.
Elements with more than 92 protons are so unstable that they don't
exist naturally, and have to be made by us in nuclear reactors.
An example is Americium-241 (95 protons), which emits a particles
and is used in smoke detectors. You probably have some of this in
your house!
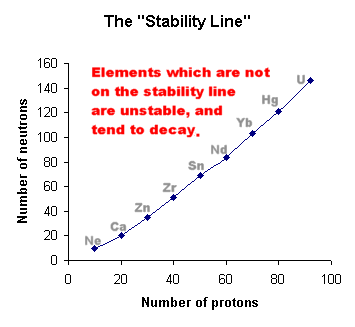
| When we plot a graph of "Number of neutrons"
against "Number of protons", we find that stable
elements lie on a "stability line".
Elements which are not on this line are unstable,
and we find that they tend to undergo alpha-decay or beta-decay
and get closer to the stability line.
They may take several steps in order to achieve
this, thus we observe decay chains for most radioactive
elements.
Radioactive decay is totally spontaneous.
There is no way to tell if an individual atom is about to
"pop", nor is there any way to predict when it's
going to do it.
However, when we're dealing with huge numbers of atoms, you
can confidently predict how many will decay on average in
any given period of time. |
 |
| Remember:-
- the atomic number of an atom is the number
of protons in its nucleus,
- the atomic mass is the number of protons
+ neutrons in its nucleus
(also called the "atomic weight" or "relative
atomic mass").
At GCSE level, we measure it in "atomic mass
units", where protons and neutrons both have
a mass of 1.
Example:- Lithium has 3 protons and
4 neutrons.
So its atomic number is 3, and its atomic mass is 7 (=3+4)
We write
it as 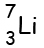
|
|
|
|

