 |
Gamma rays (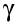 )
are electromagnetic waves, rather like X rays and radio waves. )
are electromagnetic waves, rather like X rays and radio waves.
Thus gamma rays have no mass and no charge.
You can find out more about electromagnetic waves from http://www.darvill.clara.net/emag/index.htm
After a nucleus has emitted an 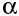 -particle
or a -particle
or a 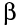 -particle,
it may still have too much energy: we say it is in an "excited
state". -particle,
it may still have too much energy: we say it is in an "excited
state".
It can get rid of this energy by emitting a
pulse of very high frequency electromagnetic radiation, called
a gamma ray. |
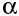 -particles
and -particles
and 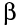 -particles
pull electrons off atoms as they pass (we say the ionise
the atoms), but -particles
pull electrons off atoms as they pass (we say the ionise
the atoms), but 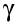 rays
don't. This means that they do not lose much energy as they travel,
as they do not interact as much with the matter they pass. rays
don't. This means that they do not lose much energy as they travel,
as they do not interact as much with the matter they pass.
Therefore, gamma rays have a high penetrating power, and
a very long range.
It's worth noting that there is no such thing as a pure 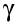 -ray
source. Gamma rays are given off by most alpha emitters and beta
emitters. If we want a source of pure gamma rays, we can get it
by using a substance that emits both beta and gamma, and simply
keep it in an aluminium container that stops the beta particles. -ray
source. Gamma rays are given off by most alpha emitters and beta
emitters. If we want a source of pure gamma rays, we can get it
by using a substance that emits both beta and gamma, and simply
keep it in an aluminium container that stops the beta particles.
Useful gamma sources include Technetium-99, which is used as a
"tracer" in medicine. This is a combined beta
and gamma source, and is chosen because betas are less harmful to
the patient than alphas (less ionisation) and because Technetium
has a short half-life (just over 6 hours), so it decays away quickly
and reduces the dose to the patient.
Remember, in Gamma decay:-
- atomic number unchanged
- atomic mass unchanged.
|
|
|
|
 |

