|
|
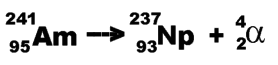
So Americium-241 (an The equation would look like this:- Note that an alpha particle is the same as the nucleus
of a Helium atom (2 protons and 2 neutrons). Alpha-decay occurs in very heavy elements, for example, Uranium and Radium. These heavy elements have too many protons to be stable. They can become more stable by emitting an alpha particle. Alpha particles have a large charge(+2), so they easily ionise other atoms that they pass. Ionising atoms requires energy, so alpha particles lose energy rapidly as they travel. Thus they have a range of only a few centimetres in air.
|
|
Can't see the menu? Try here: Home | Types BG info Alpha Beta Gamma | Sources | Uses | Dangers | Ionisation | Detecting | Measuring | Half life | Famous people | Main Quiz | Exam questions | |