 |
A Beta particle is the same as an
electron.
It has a charge of -1, and a
mass of around 1/2000th of a proton.
But wait a minute! If a nucleus contains protons
and neutrons, what's an electron doing coming out of a nucleus?
To answer this, we need to know more about protons
and neutrons:
Protons & neutrons are made of combinations
of even smaller particles, called "quarks". Under
certain conditions, a neutron can decay, to produce a proton
plus an electron. The proton stays in the nucleus, whilst
the electron flies off at high speed.
|
| This means that
when a nucleus emits a 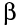 -particle: -particle:
- the atomic mass is unchanged
- the atomic number increases by
1.
This is because a neutron has changed into a
proton (almost the same mass - we can ignore the tiny mass
of the electron) and thus the number of protons has gone up.
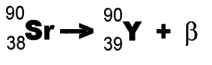
Example: Strontium-90 undergoes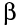 decay
and forms Yttrium-90. decay
and forms Yttrium-90.
 |
This isn't the whole
story - an almost massless particle called an "anti-neutrino"
is also emitted. Furthermore, we are only considering
"beta-minus" emission (negatively-charged
electrons).
There is another type of beta decay, called "beta-plus",
where a positively-charged electron (called a "positron")
is emitted, along with a neutrino.
Don't worry about this right now, this is way beyond
what's required for GCSE, and takes us into 'A' level
physics concepts such as antimatter. |
|
Beta decay occurs in very "neutron-rich" elements,
for example, Strontium-90 and Iodine-130. These elements are typically
created in nuclear reactors.
These elements have too few protons and too many neutrons to be
stable. They can thus become more stable by emitting a beta particle.
Beta particles have a charge of -1, and weigh only a tiny fraction
of a neutron or proton. As a result, 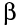 particles interact less readily with other atoms than alpha particles.
particles interact less readily with other atoms than alpha particles.
Thus beta particles cause less ionisation than alphas, and have
a longer range, typically a few metres in air.
Remember, in Beta decay :-
- atomic number increases by one
- atomic mass unchanged.
|
|
|
|



